40+ How To Calculate Molar Solubility From Ksp
AgBr s Ag aq. Determine the K sp of silver bromide given that its molar solubility is 571 x 10 7 moles per liter.

Acid Base Equilibria And Solubility Equilibria Ppt Download
305K views 1 year ago New AP General Chemistry Video Playlist This chemistry video tutorial provides a basic introduction into Ksp - the solublity product constant.
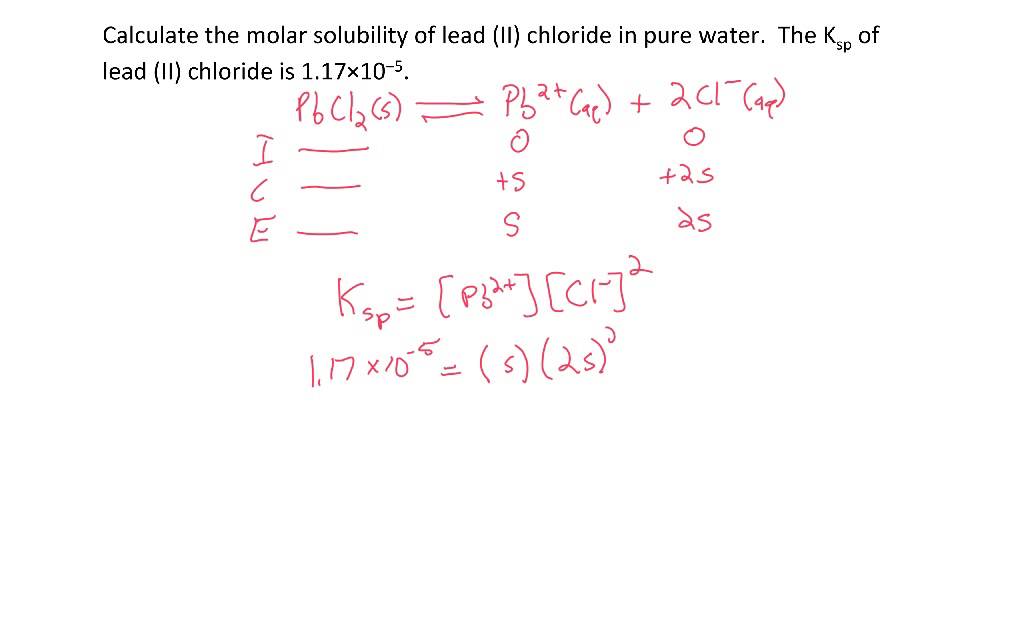
. Then 2S the solubility. The Ksp of copper I bromide CuBr is 63 10 9. The Ksp expression is.
Calculate the concentration of the ions using. Solution The solubility product constant of copper I bromide is 63 10. The latent heat of fusion of water at 0οC 6025KJmol-1 Cpm Water 573 JK-1mol-1.
K sp 0015900318 2 161 x 10-5. Tabulate the initial conditions. Determine the dissociation equation of the ionic compound.
To do so first prepare an ICE Initial Change and Equilibrium table showing the. Calculating the Solubility of an Ionic. You will also calculate.
The equilibrium for the salt is as follows. Write the equation for the compounds solubility reaction. The molar mass of a compound is the conversion factor between solubility and molar solubility.
Explains how to calculate solubilities from Ksp values. Let S the solubility saturation concentration of the Ca 2 ion in moles per liter. 1 When AgBr dissolves it dissociates like this.
Calculate the molar solubility of copper bromide. Fourth substitute the equilibrium concentrations into the equilibrium expression and solve for K sp. In this experiment you will determine the solubility product constant of CaIO32 in a saturated solution of calcium iodate.
5 The latent heat of fusion of water at 0 C is 6025 kJ mol and the molar heat capacities Pm of. Steps for Using Ksp to Calculate the Solubility of a Compound Step 1. Given that the solubility of Zn OH 2 is 42 10 -4 gL the molar solubility.
A compounds molar solubility in water can be calculated from its Kₛₚ value at 25C. You can directly assign a modality to your classes and set a due date for each class. The first thing to do is identify the values of n and m by writing the dissociation equilibrium for magnesium hydroxide MgOH2s Mg2 aq 2OH aq As you can see.
Solubility Product Constant Objective. Determine the Ksp equation from the dissociation equation.

Calculate The Molar Solubility Of Ni Oh 2 In 0 10m Naoh The Ionic Product Of Ni Oh 2 Is Youtube

Ksp And Solubility Equilibria Ppt Video Online Download

Solubility Of Agi In 1 0m Nh3 Ksp And Complex Ion Formation Youtube

How To Calculate Ksp From Solubility Pb3 Po4 2 Youtube

Determination Of The Solubility Product Constant And Molar Solubility

Using Solubility To Calculate Solute Mass Chemistry Study Com

Solved Calculate The Molar Solubility Of Agbr In 0 035m Nabr Ksp 5 10 13
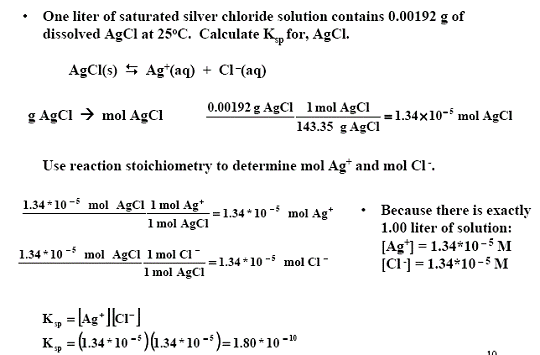
Untitled Document

Using Solubility To Calculate Solution Volume Chemistry Study Com

How To Calculate Molar Solubility From Ksp Sciencing
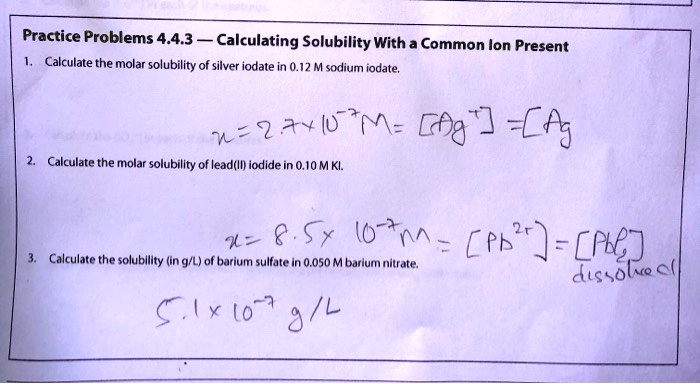
Solved Practice Problems 4 4 3 Calculating Solubility With Common Ion Present Calculate The Molar Solubility Of Silver Iodate In 0 12 M Sarlium Iodate N 2ay L M Gf Aj Calculate The Molar
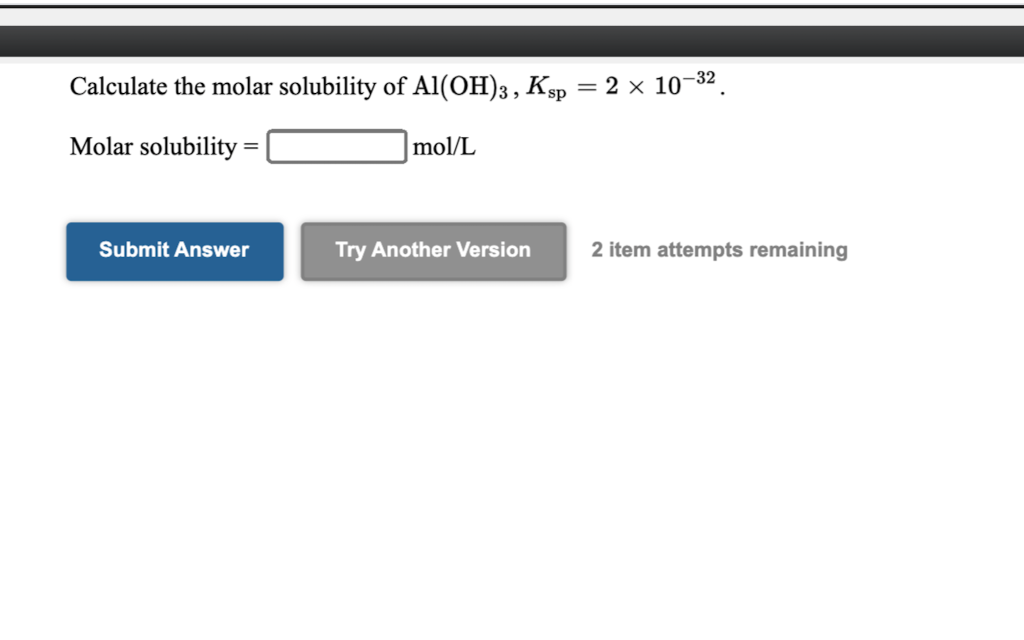
Solved Calculate The Molar Solubility Of Al Oh 3 Ksp 2 X Chegg Com
Solubility Product
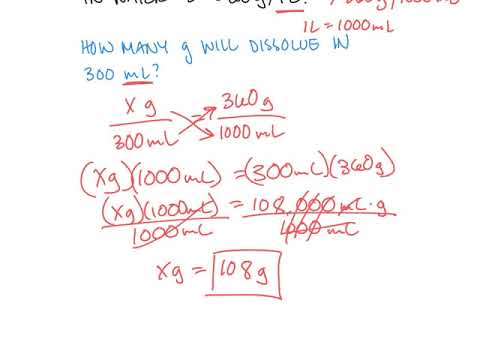
Solubility Calculations Youtube

Calculate Solubility From Ksp Youtube

What Is Ksp Solubility Product Constant Youtube
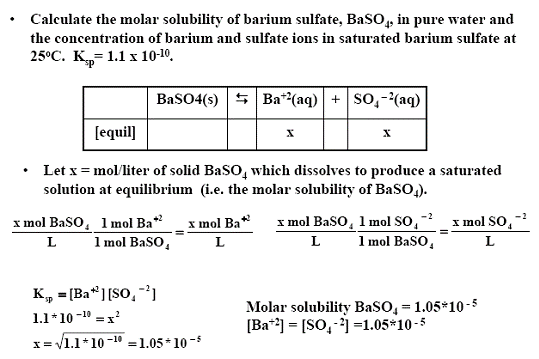
Untitled Document